What Is Water Molecule Best Described as
The water or hydrologic cycle describes the pilgrimage of water as water molecules make their way from the Earths surface to the atmosphere and back again in some cases to below the surface. It is called H2O because it has two atoms of hydrogen H and one atom of oxygen O.

Water Molecules Poster Zazzle Com Water Molecule Molecules Chemistry Classroom
5 What is the water cycle best described as.

. Using energy an enzyme removes a hydroxyl group from the carboxyl group of one amino acid and a hydrogen atom from the amino group of another amino acid to join the amino acids in a dehydration synthesis reaction that releases water. All compounds are molecules. In water since we have two single bonds we have one sigma bond each and no pi bonds.
This imparts partial negative charge to the oxygen atom and partial positive charge to hydrogen atoms. What is the diatomic nitrogen molecule best described as. Written as H2O on this website.
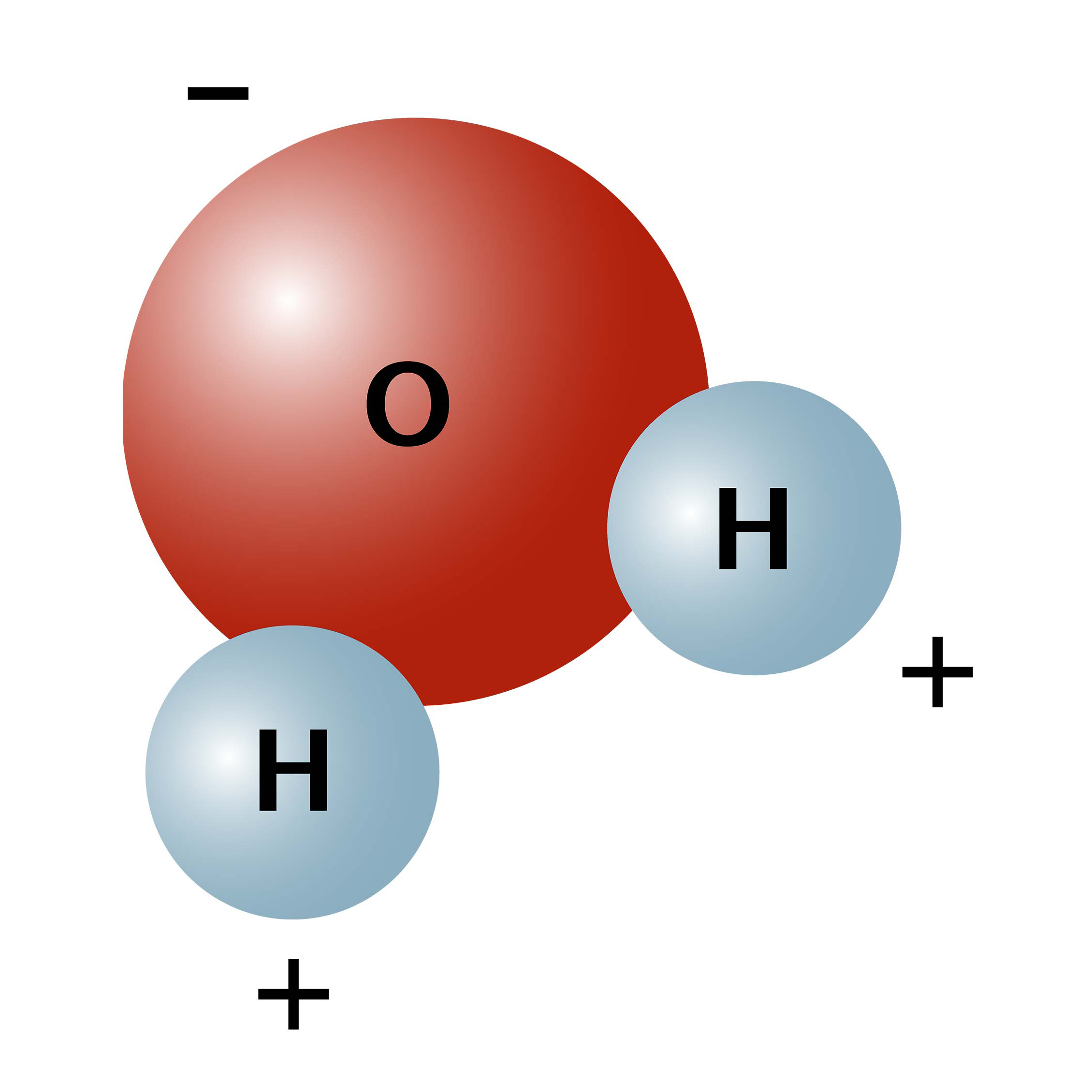
Water H2 O is a polar inorganic compound that is at room temperature a tasteless and odorless liquid nearly colorless with a hint of blueThis simplest hydrogen chalcogenide is by far the most studied chemical compound and is described as the universal solvent for its ability to dissolve many substances. Water calcium oxide and glucose are molecules that compound. Water is a polar molecule A water moleculeis formed when two atoms of hydrogen bondcovalently with an atom of oxygen.
The water molecule is very simple. Now lets take water. A mingling of molecules andor ions.
Written the 2 after the H is written in subscript as you can see in the. 9 Which of the following. The scientific name for water is H2O.
In a covalent bond electrons are shared between atoms. It has the chemical formula H 2 O meaning that one molecule of water is composed of two hydrogen atoms and one oxygen atom. Its chemical formula is H2O or less.
Banner above but due to formatting restrictions it will simply be. But this leaves us with 6-24 unpaired valence electrons. The molecule of water A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties and there are few molecules that are more stable and difficult to decompose than H 2 O.
A molecule is a piece of matter that contains two or more atoms. 8 Which of the following describes a characteristic of water that makes it cohesive in nature. This allows it to be the solvent of life.
When a chemical species is said to be polar this means that the positive and negative electrical charges are unevenly distributed. Water is a compound of two elements. The unequal sharing of electrons within a water molecule makes the water molecule _____.
Water is a polar molecule and also acts as a polar solvent. A water molecule consists of two atoms of hydrogen linked by covalent bonds to the same atom of oxygen. Therefore glucoseC6H12O6 is the molecule best described as an energy-rich molecule.
Water has many special properties. Water is vital to all plant and animal life on Earth. So we have total two sigma bonds.
6 What are the 4 stages of the water cycle. Glucose is a source of energy which is produced by green plants with the help of carbon dioxide and water in the presence of sunlight. You may say this number 0000282 µm as.
Two isotopic forms deuterium and tritium in which the atomic nuclei also contain one and two neutrons respectively are found to a small degree in water. This is a form of stored energy which is then released when oxidation of glucose takes place. Dissolving is best described as.
The tendency of an atom to pull electrons toward itself is referred to as its _____. The central atom Oxygen has a valence configuration of 2s22p4 that is 6 electrons. 3 This molecule is essential in the life of living beings serving as a medium for the metabolism of biomolecules is found.
Water H2O is an inorganic chemical compound formed by two hydrogen H and one oxygen O atoms. The diameter of a water molecule H2O is closely calculated to be about 0000282 µm micrometers millionths of a meter in diameter. What is the term for a solution with a high pH number.
The arrangement of the electron pairs around the central oxygen is tetrahedral with the non-bonding pairs taking up slightly more space than the bonding pairs but since we dont see the the electrons but only the atoms the molecule. These form two lone pairs pairs of. There are millions of these molecules in one drop of water.
Since oxygen is more electronegative as compared to hydrogen atoms the shared electrons are attracted towards the oxygen atom. Indeed water as found in nature. Most hydrogen atoms have a nucleus consisting solely of a proton.
A water molecule consists of one oxygen atom bonded to two hydrogen atoms by covalent bonds. The water molecule is composed of two hydrogen atoms each linked by a single chemical bond to an oxygen atom. The quick answer is.
Water is in fact a chemical. 7 What is the water cycle for kids. Water is found almost everywhere on earth and is required by all known life.
Commonly HOH which is what this website is named after. The most common example of this is water. Atoms are the tiniest pieces of matter.
The oxygen atom attracts the electrons more strongly than the hydrogen. The positive charge comes from the atomic nucleus while the electrons supply the negative charge. Consequently the electrons in the water molecule spend slightly more time around the oxygen atomic center and less time around the hydrogen atomic centers.
Molecules made up of two or more elements are called compounds. This gigantic system powered by energy from the Sun is a continuous exchange of moisture between the oceans the atmosphere. The central oxygen atom has 2 non-bonding pairs of electrons and 2 pairs of shared bonding electrons each to a hydrogen atom.
The molecule that is best described as glucose has the chemical formula C6H12O6. Not all molecules are compounds. Choose from the following statements the one that best describes the reactions illustrated in the figure above.
Atoms of oxygen are electronegative and attract the shared electrons in their covalent bonds. Water is a chemical compound and polar molecule which is liquid at standard temperature and pressure. Its the movement of electrons that determines polarity.
Also one of the most well-known chemical formulas. Water is a source of _____ for chemical reactions in cells. Inside a water molecule Water.
Two-hundred-Eighty-Two millionths of a micrometer. In water the sharing is not equal.
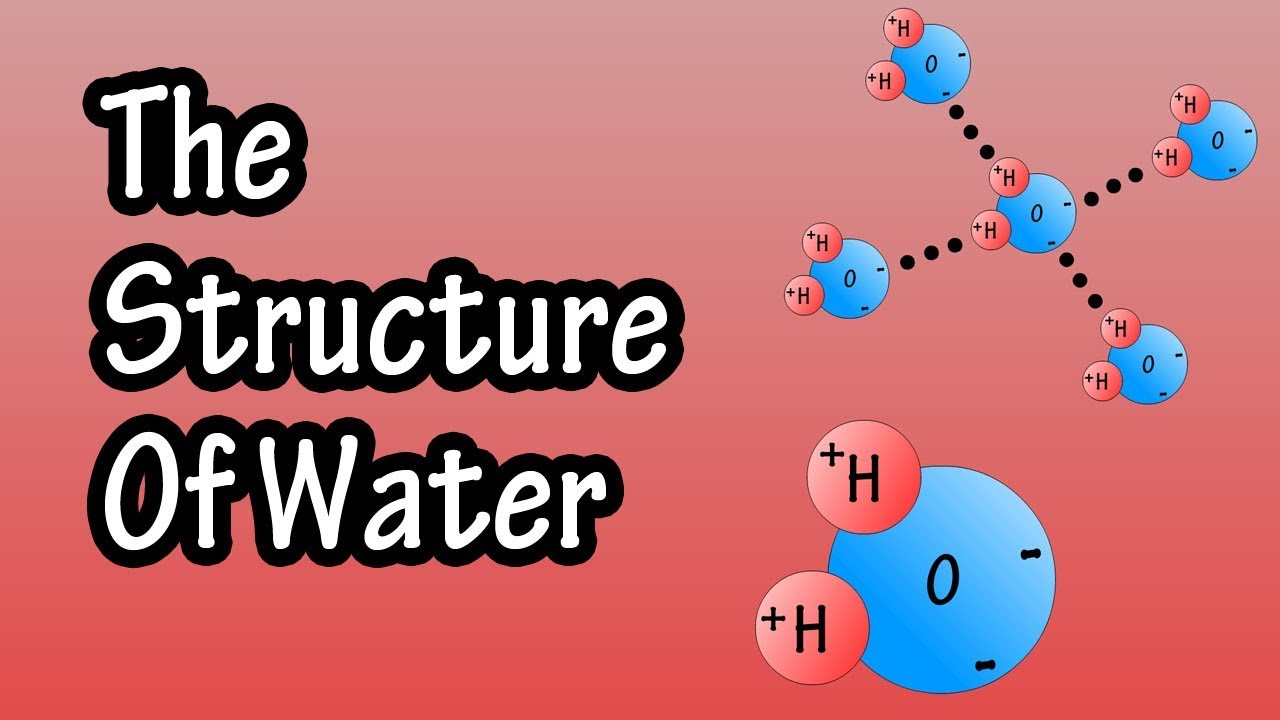
Structure Of Water Molecule Chemistry Of Water Properties Of Water Composition Of Water Youtube

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules
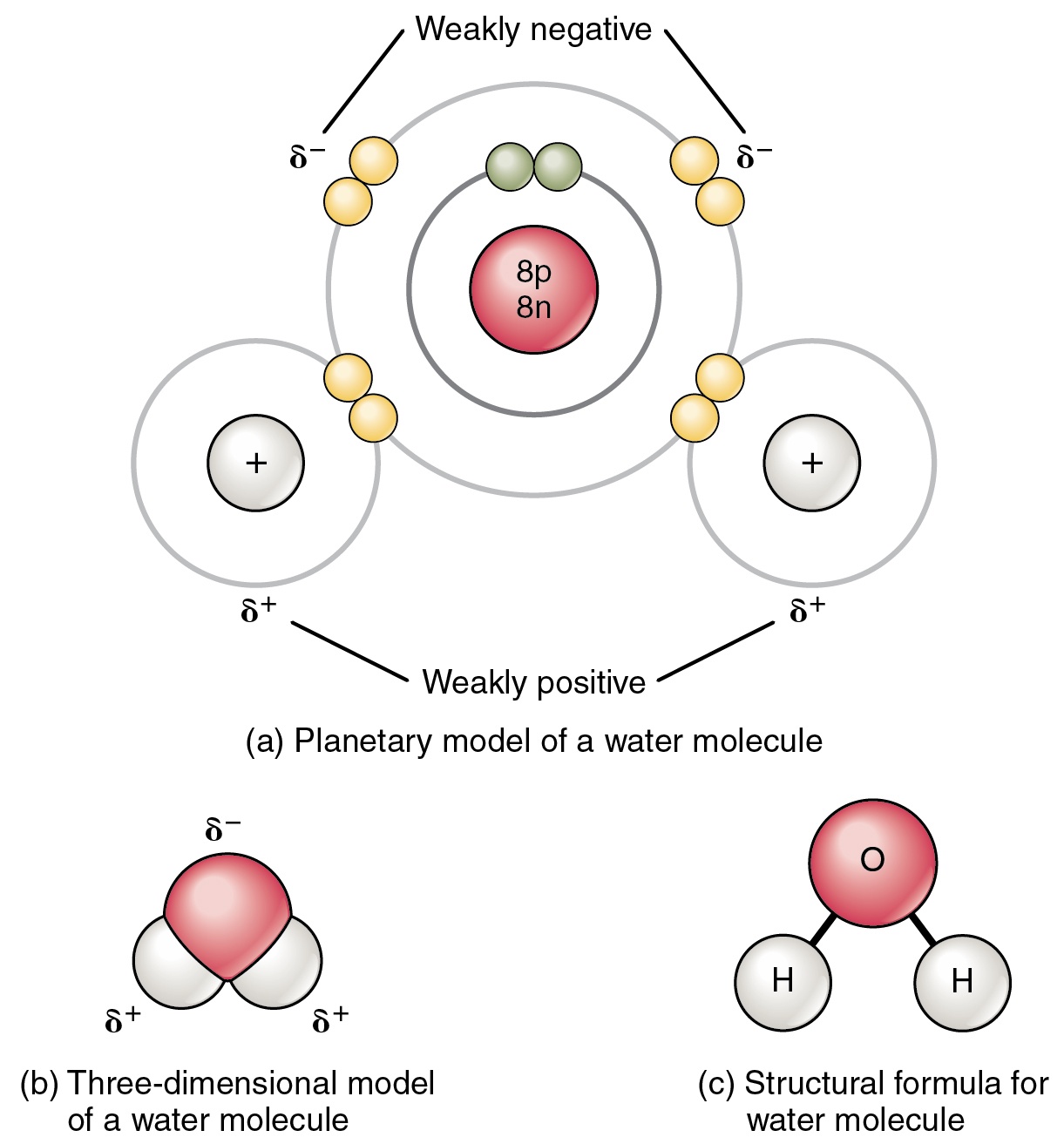
Water Molecular Structure Bonding Expii

Hydrogen Bonds In Water Article Khan Academy

Hydrogen Bonding Strongest Fon Chemie Unbelebte Natur Lebewesen
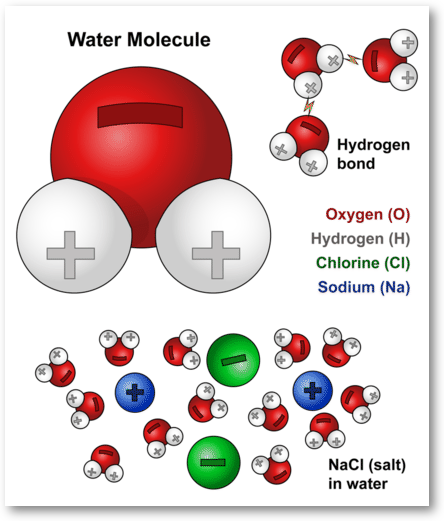
Water Molecules And Their Interaction With Salt U S Geological Survey
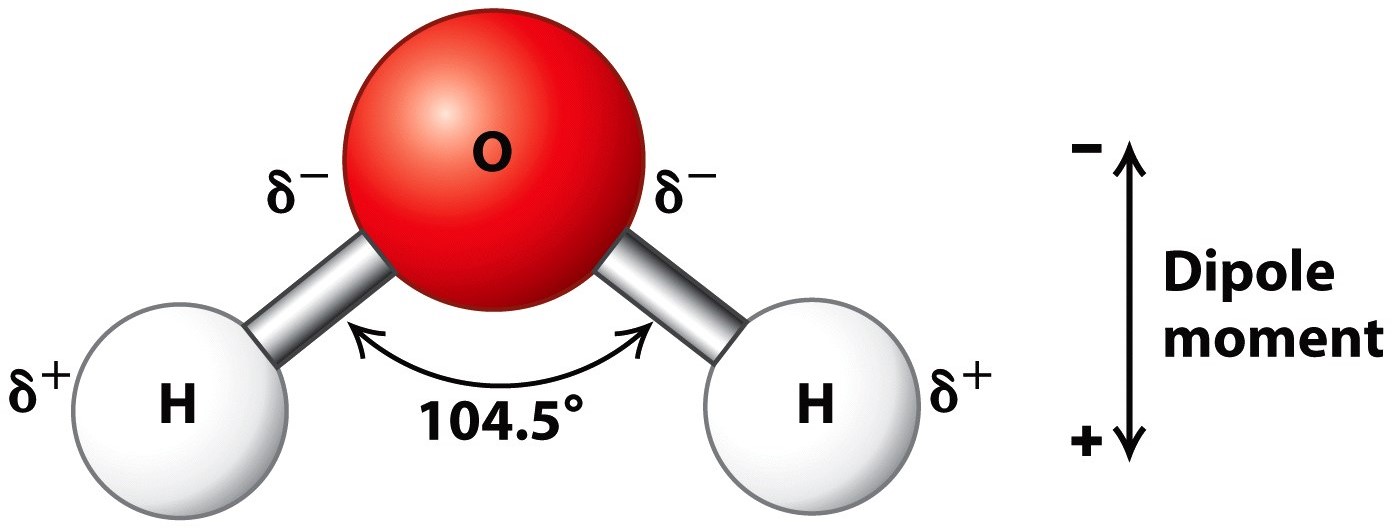
Water Facts Properties Structure Compounds Summary
First Images Of A Hydrogen Bond

The Structure And Properties Of Water Introduction To Chemistry

Worldofchemicals On Twitter Water Molecule Molecules Teaching Chemistry

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

What Are Doctors Saying Woke Water Co Video In 2021 Kangen Water Kangen Organic Chemistry Study

Lesson Summary Water And Life Article Khan Academy
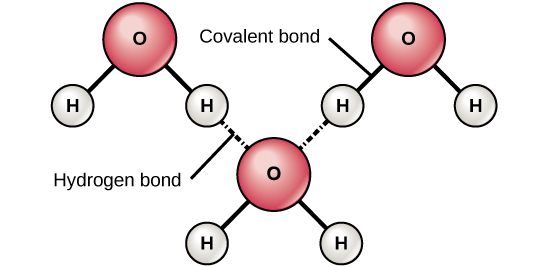
The Building Blocks Of Molecules Biology I

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

Model Of A Hydrogen Molecule Chemistry Chemistry Review Toddler Education

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules

H Bonds In Water You Tube Video Gets Good At 1 20 Hydrogen Bond Digital Learning Body Systems
Comments
Post a Comment